Hashishene, the new terpene of cannabis
List of contents
Hashish and terpenes
As we already know, terpenes are responsible for the taste and scent of many of the vegetables that produce them. They are a broad class of organic hydrocarbons derived from isoprene (CH2=C(CH3)CH=CH2) which compose the bulk of resins and essential oils of plants, thus providing unique flavours to each individual as a result of terpene combination. They are also called terpenoids when they have undergone an oxidating or molecular re-combination process. As we also know, most cannabis terpenes have properties of great medicinal value.
As the flowering stage progresses, more and more terpenes are secreted inside the trichome heads, so that the terpene profile of the plant changes as it ripens. The same thing happens when drying and curing buds, the process of oxidation and partial decarboxylation to which buds are exposed makes their terpene range to change over time. Some terpenes will degrade faster than others, so the terpene range of the weed - we must remember that we know of more than 100 terpenes in cannabis - will vary unless it is vacuum-sealed and properly stored. This fact explains why the smell and taste of one sample can evolve throughout the drying and curing process.

Probably, any concentrate lover has realized that, many times, the extraction process changes the terpene profile of the weed, so the resulting extract lacks some of the organoleptic features of the buds from which it comes. In this way, cannabis extracts have a common taste and smell - with subtle variations - regardless of the strain used to make them. This happens especially when using dried/cured plant material, since as we have already seen in our posts about Fresh Frozen and Fresh Chilled, these type of concentrates have a smell and taste much closer to those of the fresh plant material. Somehow, isolating and concentrating the resing glands leads us to limit the terpene range, so we can't properly appreciate the "personality" of each sample. But, why does it happen?
Study on the volatile compounds of cannabis sativa
A study conducted by Jean-Jaques Filippi, Marie Marchini, Céline Charvoz, Laurence Dujourdy and Nicolas Baldovini ("Multidimensional analysis of cannabis volatile constituents: Identification of 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane as a volatile marker of hashish, the resin of Cannabis sativa L.") at the end of 2014 seems to have found the answer to this question. According to these researchers, the typical "hashish flavour" of many resin extracts made from dried and oxidized buds comes from the degradation of a single terpene, which creates an unusual and rare monoterpene (5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane). The five researchers propose in their study a name for this particular monoterpene, Hashishene. While the rearrangement of myrcene and the formation of 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane was already investigated by Robert S. H. Liu and George S. Hammond in 1965 (US 3380903 A), this phenomenon had never been observed before in hashish samples.

The volatile constituents of the samples used to conduct the study (fresh buds, dried buds and hashish) were investigated using headspace solid phase microextraction (HS-SPME) and hyphenated gas chromatography techniques (GC-MS, GC×GC-MS), showing clear differences in terpene profile between weed and hashish samples, mainly resulting from photo-oxidation of the plant material during the drying and extraction processes. Thus, the hashish samples analyzed showed considerable amounts of a rare monoterpene among their volatile compounds, which came from a rearrangement of beta-myrcene during the manufacture of hashish. Moreover, the researchers claim that this monoterpene would be almost exclusive from cannabis plants, so they propose to call this new volatile marker "Hashishene".
Let's take a closer look now at what is beta-myrcene and how it degrades into Hashishene.
What is beta-myrcene?
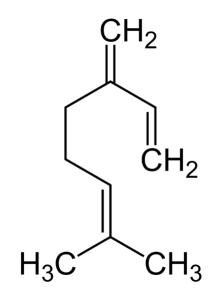
Beta-myrcene - also known as myrcene - is a natural hydrocarbon (7-Methyl-3-methylene-1,6-octadiene), more precisely one of the most important monoterpenes found in cannabis plants (it is also found in other plants like hops, parsley or bay) and widely used in the production of fragances for its pleasant scent, although it is unstable in air and tends to polymerize. Its name comes from Myrcia sphaerocarpa, a medicinal plant found in Brazil with high amounts of myrcene.
Myrcene is also a precusor to other terpenes, helping to form them, and synergizes their antibiotic properties. It is also believed to play a crucial role in the effects of cannabis. A study conducted in Switzerland in 1997 showed that myrcene was the most common terpene in 16 different cannabis strains, being over 50% the total terpene content of some of them.
Myrcene can change the permeability of cell membranes to allow more cannabinoid absorption by the brain, thus regulating the effects of other cannabinoids (in a similar way than CBD does). It is also believed that myrcene makes THC, CBD and CBG more effective. Myrcene has many therapeutic effects, being used to relax muscles and combat pain. It also has anti-depressant and anti-inflammatory properties, plus antimicrobial, antioxidant, antiseptic, and anti-carcinogen effects. It slows bacterial growth, inhibits cell mutation (a very important factor when fighting cancer), suppresses muscle spasms and is even used to treat psychosis because of its relaxing and calming effect.
Its smell is very complex, earthy, balsamic and spicy, but also reminiscent of grapes, peaches, vanilla, grass, wood and pepper. Both the smell and taste dissipate with high temperatures.
Beta-myrcene:
- Name: 7-Methyl-3-methylene-1,6-octadiene
- Formula: C10H16
- Molecular Weight: 136.23404 g/mol
- Decarboxylation Point: 115-145°C (239°F to 293°F)
- Boiling Point: 168°C (334°F, 442ºK)
- Critical Pressure: 2421.88 kPa
- LD50 (Lethal Dose): >5g/kg
Therapeutic uses of myrcene:
- Analgesic – Pain relief
- Antibacterial – Slows bacterial growth
- Anti-Diabetic – Helps mitigate the effects of diabetes
- Anti-inflammatory – Systemic reduction of inflammations
- Anti-Insomnia – Aids with sleep disorders
- Anti-Proliferative/Anti-Mutagenic – Inhibits cell mutation, including cancer cells
- Antipsychotic – Tranquilizing effects, relieves symptoms of psychosis
- Antispasmodic – Suppresses muscle spasms
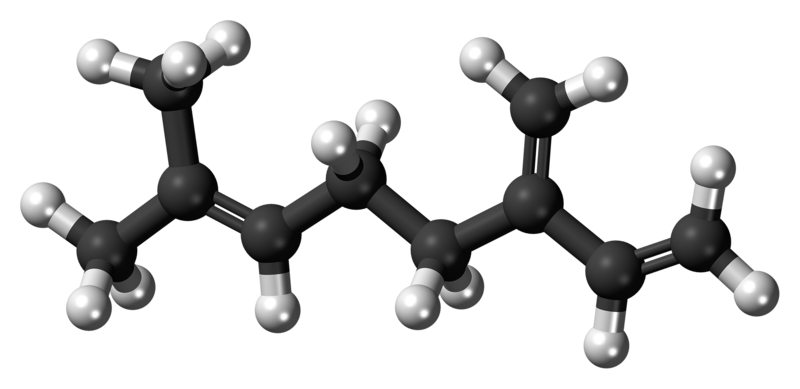
Hashishene as rearrangement of beta-myrcene
We know that cannabis contains high amounts of monoterpenes and sesquiterpenes. Alpha and beta-pinene, beta-myrcene and limonene are the most commonly found monoterpenes, while the predominant sesquiterpenes are beta-caryophyllene and alpha-humulene. Seven out of ten cannabis samples used in this study contained large amounts of beta-myrcene (19,5-28,7%), but what about the hashish samples?
After a first series of analyses, researchers found out that every hashish sample contained remarkable amounts of an unusual monoterpene - 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane - eluting before alpha-pinene. This finding was quite surprising since this compound had only been isolated once before this study - although it was first identified in Boswellia species - and it was as a constituent of the essential oil of Mentha cardiaca L. Moreover, and on a second series of analyses, "hashishene" was found in almost all hashish samples among the most abundant apolar constituents (1,1-14-9%), while it was detected in samples of dried and fresh herb in very low amounts. A total of 186 constituents were identified in these analyses.
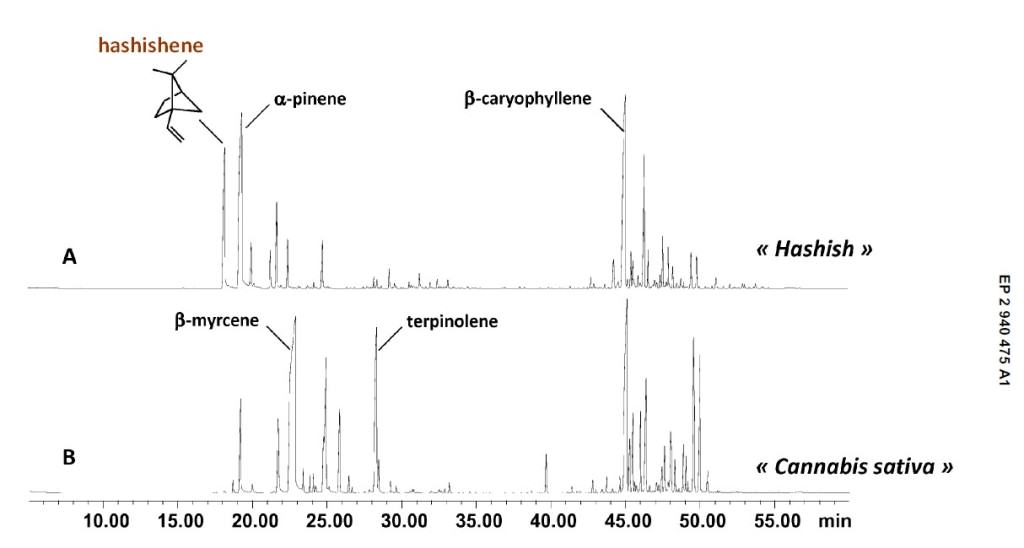
Before this study, beta-myrcene was already considered as one of the most abundant volatile constituents of cannabis, and has been proposed as specific marker of marijuana. According to the results of this study, the presence of "hashishene" would be directly linked with the high amounts of beta-myrcene detected in fresh cannabis buds. Researchers state that exposure to sunlight would be one of the factors related with the formation of hashishene from beta-myrcene, supporting the photolytic origin of "hashishene".
All hashish samples showed a wider diversity in oxygenated terpenes than cannabis buds, and many alcohols, aldehydes and ketones could be related to the hydrocarbons found in cannabis. They observed that many oxygenated derivatives actually came from the initial terpenes, more specifically from a photo-oxydation process. In this way, terpene photo-oxidation leads to the formation of allylic hydroperoxides, which will soon generate alcohols when losing an oxygen atom.
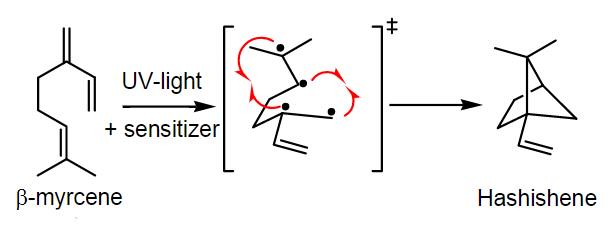
Other volatile constituents widely detected in most hashish samples were caryophyllene/humulane derivatives - as well as linear alkanes, esters, fatty acids or alcohols - all as a result of processes of isomerization, dehydration, cyclisation and photo-oxidation from the manufacture of hashish. Researchers propose to rename monoterpene 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane - a photolytic rearrangement of beta-myrcene - as "hashishene" due to its rare occurrence in other essential oils and to its high abundance in hashish.
Hashishene:
- Name: 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane
- Formula: C10H16
- Molecular Weight: 136.234 g/mol
- Boiling Point: 161°C (321°F, 434ºK)
- Critical Pressure: 3022.28 kPa

You can take a look at this study in this link.