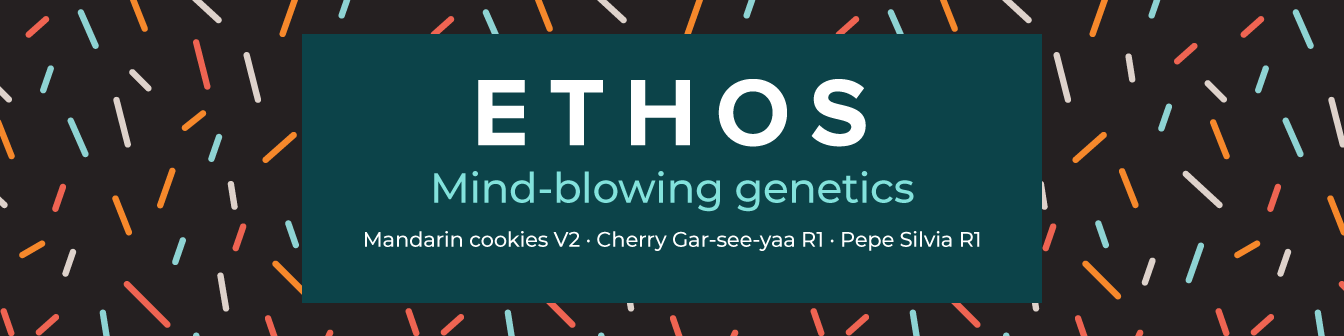
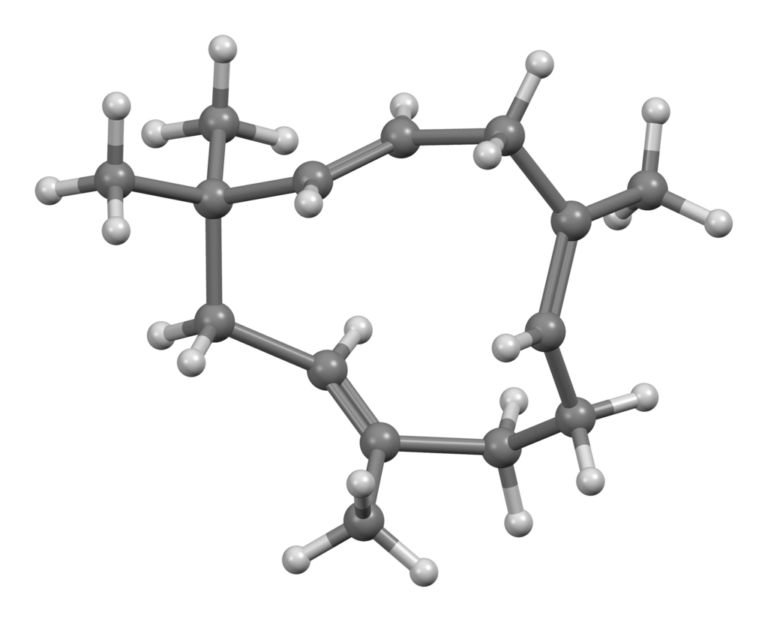
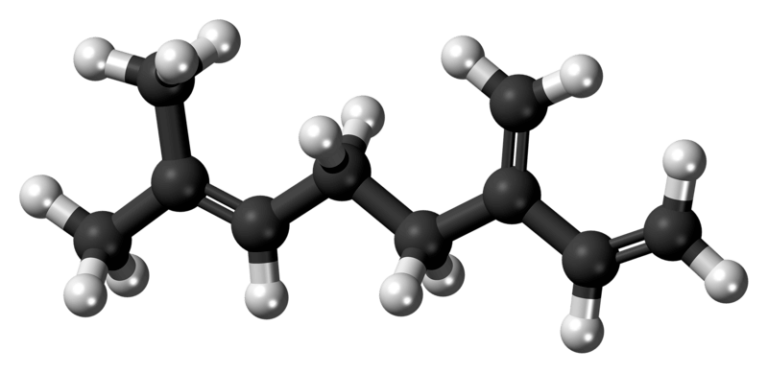
Limonene: An essential terpene in Nature
List of contents
What is Limonene?
- Name: Limonene, d-limonene
- Formula: C10H16
- IUPAC Name: 1-methyl-4-(1-methylethenyl)-cyclohexene
- Density: 841.1 kg/m3
- Molar mass: 136.23 g/mol
- Boiling point: 176ºC/348.8ºF
- Presence at room temperature: Colourless liquid, very low solubility in water
Limonene is one of the most widely used monoterpenes - aromatic molecules produced by a large number of plants - in the industry, either to make food or as constituents of perfumes, medicines, or detergents, also to manufacture biodegradable solvents or to replace toxic solvents. It is a terpene commonly related to cannabis since many of the existing cannabis strains contain limonene to a greater or lesser degree. Citrus fruits also contain high amounts of limonene, especially in the peel, as well as other plants like mint, rosemary, or juniper.
It is usually classed among limonoids along with other terpenes like pinene or eucalyptol, which are often found together in the same fruits. The D-isomer (also called R or alpha) has an intense citric aroma, reminiscent of oranges or lemons, while the L-isomer (also called S or beta) is closer to pine trees. Limonene is used in the industry to mask other odors or flavors, and - along with camphene - can be obtained by catalyzing another well-known aromatic compound, pinene. The most widely used methods to extract limonene - and other terpenes - from fruits are steam distillation or centrifugal force.
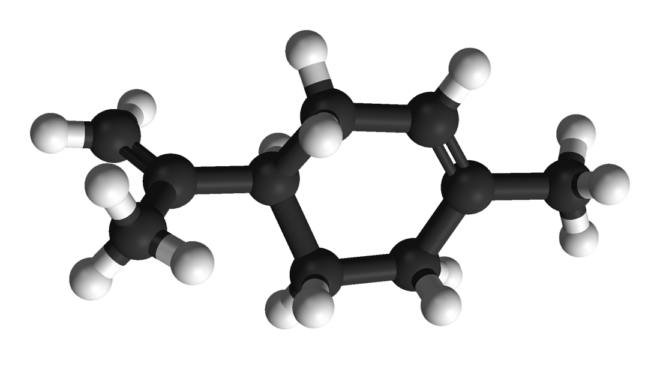
Synthesis of limonene
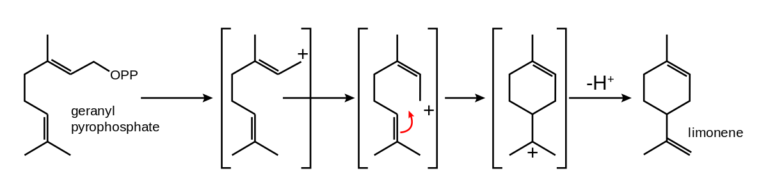
As happens with other monoterpenes (terpenes with 10 carbons) present in cannabis, limonene comes from geranyl pyrophosphate. However, as we already know, not all cannabis plants produce the same amount of each cannabinoid or terpene, so every plant has its own, unique profile. Limonene is, in turn, the precursor of a terpenoid called carvone (found in juniper and peppermint, among others) and also of perillyl alcohol and P-cymene, which is obtained by catalytic dehydrogenation of limonene and shares a multitude of uses with it in the industry. Limonene oxidizes when it comes in contact with air, producing carveol, limonene oxide, and the aforementioned carvone, as well as other potent allergens.
It is believed that plants produce limonene as a natural insecticide, which repels insects thanks to its intense smell. Limonene is the second most found terpene in Nature (after pinene, which is the first) which suggests that it is a compound with enormous potential. Besides other plants, it is mostly found in lemons, limes, oranges, mandarins, or grapefruits.
The smell and taste of limonene
While this point may seem obvious, the truth is that the smell and taste of limonene vary depending on each isomer. The scent of D-limonene is closer to oranges, with sweet and lemony notes. On the other hand, the L-isomer is reminiscent of pine trees with citric and solvent notes.
Properties and effects of limonene
The medicinal properties of this cyclic carbohydrate are numerous and well-known. Besides its applications in the medical field, it is also being increasingly used in the industry to replace toxic substances. Among its many uses, the following are highlighted for their multiple applications and the numerous studies proving their efficacy:
- Industrial biodegradable solvent for resins, dyes, greases, oils or pigments
- Recyclable solvent for extractions (it has been used to replace hexane when extracting oil from rice bran)
- Cleaning of sewers and drains
- Manufacturing of paints and glues
- Flavoring agent for foods
- Flavoring excipient for medicines (it masks the bitter taste of alkaloids)
- Aromatic component in perfumes and cosmetics
- Insect repellent (especially the D-isomer)
- Synthesis of other compounds

Furthermore, limonene also has a number of medicinal properties of great interest and potential, such as:
- Anti-cancer effect (this molecule increases the levels of hepatic enzymes involved in the detoxification of carcinogens, promoting the activity of the GST system. It also metabolizes carcinogenic substances into less toxic compounds)
- Tumor reduction in mammals (anti-inflammatory properties)
- Triggering of entourage effect with cannabinoids like THC-A, CBD-A, CBC-A, CBC, CBG, or terpenes like linalool or caryophyllene by reducing the permeability of the skin, digestive tract, and mucous membranes
- Anxiolytic/anti-depressive effect (widely used in aromatherapy to treat stress and improve mood)
- Antiseptic properties
- Anti-bacterial and anti-fungal agent
- Tissue regeneration (damaged skin, acne, athlete's food)
- Ulcer and acid reflux treatments
- Treatment of gallstones and kidney stones
The toxicity of limonene is low; adverse reactions after using it are uncommon, although cases of irritant reactions on the skin and respiratory system after prolonged industrial exposure may appear. Still, it is considered a safe compound for humans.

Cannabis strains with high limonene content
The number of genetics with considerable amounts of limonene is huge, especially if we focus on Sativa varieties. The scent and taste of this terpene is highly appreciated by many cannabis users since it creates a wide range of fruity and citric hints. The following strains usually reach high limonene content:
- Lemon OG Candy by Philosopher Seeds
- SFV OG Kush IBL by The Cali Connection
- Lemon Tree by Barney's Farm
- Lemon Shining Silver Haze by Royal Queen Seeds
- San Fernando Lemon Kush by Sweet Seeds
Without a doubt, limonene is one of the most important terpenes in Nature, with endless applications in both the industrial and medical fields. It can be easily extracted and has a large number of properties, which makes it a widely used compound in different industrial sectors. Furthermore, its amazing therapeutic and organoleptic properties give this compound a promising future with regard to cannabis breeding.
Happy growing!
Studies and publications on limonene consulted for the writing of this article:
- Skin repair properties of d-Limonene and perillyl alcohol in murine models. d'Alessio PA, Mirshahi M, Bisson JF, Bene MC
- Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Miller JA, Lang JE, Ley M, Nagle R, Hsu CH, Thompson PA, Cordova C, Waer A, Chow HH
- Plasma metabolomic profiles of breast cancer patients after short-term limonene intervention. Miller JA, Pappan K, Thompson PA, Want EJ, Siskos AP, Keun HC, Wulff J, Hu C, Lang JE, Chow HH
- d-Limonene: a bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Jessica A. Miller, Patricia A. Thompson, Iman A. Hakim, H.-H. Sherry Chow, Cynthia A. Thomson
- Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. Hirota R, Roger NN, Nakamura H, Song HS, Sawamura M, Suganuma N.
- Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. de Almeida AA, Costa JP, de Carvalho RB, de Sousa DP, de Freitas RM.
- Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. Bharat Singh, Ram A. Sharma
- Essential oil of Cannabis sativa L. strains. V. Mediavilla, S. Steinemann
- Concise international chemical assessment document nº5, Limonene. Dr A. Falk Filipsson, Mr J. Bard, Åseda, Ms S. Karlsson
- Air oxidation of d-limonene (the citrus solvent) creates potent allergens. Ann-Therése Karlberg, Kerstin Magnusson, Ulrika Nilsson